- Gold 997.08
- Silver 15.30
- Palladium 347.38
- Platinum 413.28

- Frontpage
-
New
Tilbage
Luk -
Tools & Machines
Tilbage
LukTools & Machines
-
Se alt i Tools & Machines
- New
-
Hand Tools
Tilbage
LukHand Tools
-
Se alt i Hand Tools
- Pliers
- Saw Frames & Blades
- Precisions Files / Handles for needle files
- Calipers / Measuring
- Dividers / Engineers Squares
- Magnifier / Glasses
- Shears
- Tweezers
- Soldering Tweezers / Third-hand
- Hammers
- Ring Sticks / Ring Gauges / Wrist Meters
- Joint Cutters / Profile Cutters
- Special Tools
- Reamers / Pin Vices
- Stamps
-
Drills / Cutters / Miniature brushes
Tilbage
LukDrills / Cutters / Miniature brushes
-
Grinding / Polishing
Tilbage
LukGrinding / Polishing
-
Engraving / Stone Setting
-
Metal Forming
Tilbage
Luk -
Soldering / Melting Equipment
Tilbage
Luk -
Machines
Tilbage
LukMachines
-
Se alt i Machines
- Electropolishing
- Electroplating
- Engraving Machines / Laser Engraving
- Compressors
- Magnetic Tumbler / Rotary Tumbler
- Micromotors / Flex Shaft motors
- Ring Stretchers / Ring Bending Machines
- Blasting
- Grinding / Polishing / Brass
- Pickling Units
- Draw Bench
- Extraction unit
- Ultrasonic Cleaners / Steam Jet Cleaners
- Rolling Mills
- Vivacolor
-
Wax / Casting
-
Literature
Tilbage
LukLiterature
-
Equipment
- Watchmaker Tools
-
Chemicals / Cleaning
Tilbage
Luk
-
Findings
Tilbage
LukFindings
-
Se alt i Findings
- New findings
-
Pearl Clasps / Parts
Tilbage
Luk -
Settings / Pendants
Tilbage
LukSettings / Pendants
-
Brooch
Tilbage
Luk -
Ear Parts
-
Cufflinks / Tie Clips / Tie Slides / Button Backs
Tilbage
LukCufflinks / Tie Clips / Tie Slides / Button Backs
-
Clasps
-
Chains
Tilbage
Luk -
Assorted Findings
Tilbage
Luk
-
Diamonds & Gemstones
Tilbage
LukDiamonds & Gemstones
-
Courses
- Log ind
- Min konto
- Log ud
-
Vælg sprog
Tilbage
LukVælg sprog
Guide to the Periodic Table
You may be familiar with O, H, and Ag as elements from the periodic table.
However, there are actually up to 118 elements to navigate through. The periodic table displays all the elements that scientists have discovered so far.
Here's a brief overview of what the system is, how to read it, and how to use it.
The inventor of the Periodic Table
The periodic table was invented by the Russian chemist Dmitri Mendeleev in 1869. At that time, only 69 elements were known. Since then, many more have been discovered, and scientists worldwide are striving to find the next element, number 119.
Mendeleev discovered that every seventh element shares common properties. He organized the elements into 18 groups based on similarities in the chemical behavior of substances, which make up the vertical columns in the system. Additionally, the elements are arranged in seven periods horizontally, indicating how many electron shells the elements have around them.
He, however, left gaps as there were elements that had not yet been discovered. Subsequently, more elements were found that fit into the Russian chemist's table.
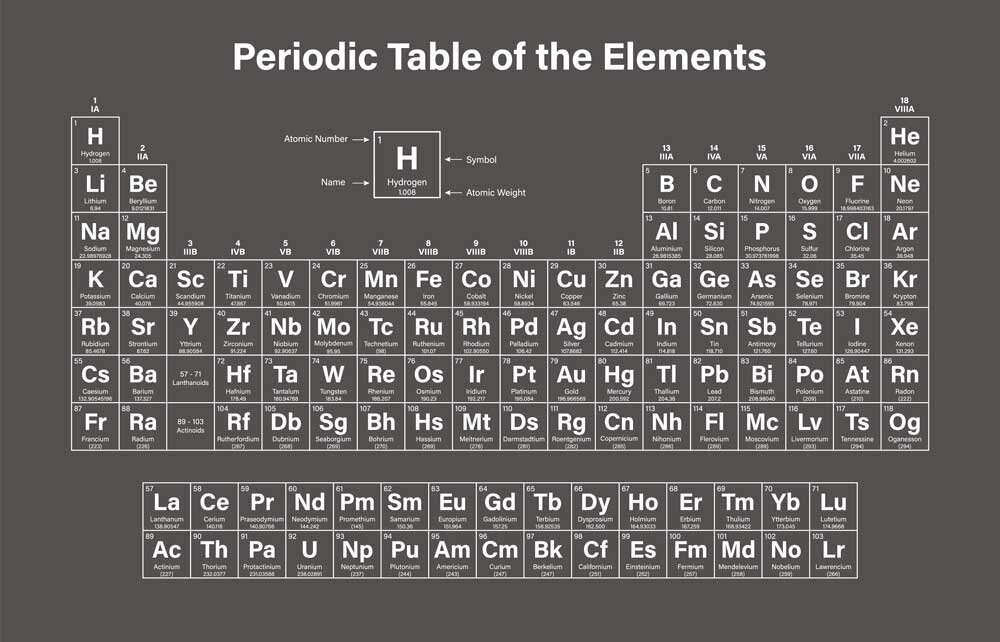
How to read the Periodic Table
The periodic table is divided into 18 vertical groups, which share chemical properties. For example, Group 1 consists of alkali metals, which are soft and malleable metals, highly reactive (they can, in some cases, react violently with water), while Group 18 contains noble gases.
Horizontally, there are "periods," indicating how many electron shells the elements have.
Additionally, the different elements are indicated with colors based on their physical state: gas, liquid, solid, or unknown.
The meaning of the numbers
If you take a quick look, you'll notice that each individual element is numbered, starting from 1 (H – hydrogen) and going up to element number 118.
The atomic number determines the number of protons in the nucleus. Therefore, the lightest element is hydrogen, with one proton in the nucleus. The heaviest naturally occurring element is number 94, plutonium, which has 94 protons.
The first 94 elements exist naturally, while elements 95 to 118 have been synthesized in laboratories, nuclear reactors, or through nuclear explosions.
These numbers trace back to Niels Bohr, who, in 1913, proposed his atomic theory. This theory provided the first theoretical explanation that an atom is constructed with a nucleus containing protons and neutrons.
What is the Periodic Table used for?
The periodic table enables an overview of the various elements and their interactions. Our understanding of the compounds and interactions of elements allows for the development of new technologies, foods, materials, and more.
For example, consider table salt, a harmless chemical compound composed of an otherwise highly explosive metal (element number 11) and toxic chlorine gas (element number 17).
Many high-tech products in our daily lives also leverage properties of specific elements, such as various electronics (silicon), smartphones (tantalum), and fuel cells (platinum). In this way, the periodic table aids in comprehending and harnessing the diverse properties of elements to create novel and innovative products and solutions.
Shop online
As a customer of Aktiv Guld, you can quickly and easily make your purchases online, regardless of whether it is from a computer, smartphone or tablet.
Login >
If you are not already registered as a user, you can do so here: